School of Medicine Fellowship Proposal Development Routing Form (PDRF)
Scroll through the steps below for guidance on how to initiate and complete a School of Medicine Fellowship PDRF, or use the quick links below to view guidance on a specific PDRF page.
- Initiate a New SoM Fellowship Proposal PDRF
- PDRF Screen Navigation
- Fellow & Project Location
- Admin & Sponsor Details
- Budget Questions
- Budget Details
- Project Questions
- Attachments
- Approvers & Comments
- Emails
- Review for Completeness & Routing
Initiate a New Fellowship Proposal - Proposal Development Routing Form (PDRF)
- Log into the Stanford Electronic Research Administration system at https://sera.stanford.edu/ with your SUNet ID and password
- Depending on your SeRA access, the right side of your SeRA home (landing) page displays either a…
- Start Proposal teal button. Click here, and then select PDRF from the drop-down menu

OR
- Start PDRF teal button. Click here
- Select the SoM Post-Doc Fellowship from the drop-down menu options

A. Click No, or bypass the question entirely (as No is a default answer) to the question of "Has a Cayuse application been started for this proposal?". Click Start.

PDRF Screen Navigation

Left-hand Navigation Menu (LHN):

- Home – Takes you back to your SeRA home page
- SPO - ###### - Takes you to the SPO level/Project Summary page of the SeRA record
- Proposal (P#) – Takes you to the Proposal Summary page for this proposal
- PR###### – Takes you the transaction home screen of this PDRF
- Transaction Home – Takes you the transaction home screen of this PDRF
- PI & Project Location 🡪 Approvers & Comments – You can move between the pages of the PDRF by utilizing this left-hand navigation (LHN) menu or by clicking on the Previous or Next buttons located at the bottom right of your screen on each page of the PD

- Emails – You can send an email to SeRA (with some added “tags” in the subject line), and the SeRA system will automatically attach your email (and any attachments in the email) to the appropriate project or agreement in SeRA
Right-hand Navigation Menus (RHM):
Actions:


- Save – Saves your work
- Reassign – Laterally moves the PDRF i.e., to another department research administrator- DO NOT use this to assign a PDRF to your Institutional Official
- Review for Completeness – View Project Comments (number in parenthesis reflect number of comments)
- Submit for Approval – Routes PDRF for approvals
- Terminate – Terminates PDRF transaction
NOTE: Before routing a PDRF, SeRA will always auto check for completeness. If any required information is missing, you will be notified and required to complete the requisite fields before the PDRF will route successfully.
Quick Links:

- View PDRF – View a PDF summary of the PDRF
- Add Project Comments – Add comments that are visible on the front page of the SPO record. Anyone with SPO access will be able to view project comments.
- View Project Comments (number in parenthesis reflect number of comments)
- Add Attachments – Allows user to add attachments directly to the PDRF level of the SeRA SPO record of the project
- View Project Attachments – Allows user to see all SPO record attachments for this project
- View Related SPO/ RRA (number in parenthesis reflect number of Related SPO/ Research Related Agreements)
Fellow Project Location
- In the Fellow field, key in the first 3 letters of the PI’s SUNet ID or name to populate and select from the name list.
- Searching and selecting by SUNet ID is highly recommended

- Once a Fellow is selected, their contact email will display in addition to a quick link to their StanfordWho information

- Select the Fellow Status (Predoctoral, Postdoctoral, Prospective, Other)

- Registered at Stanford University, select Yes or No from pick-list

- Select appropriate Citizenship status

- In the Faculty Mentor field, key in the first 3 letters of the Faculty Mentor’s last name or SUNet ID to populate and select from the name list
- Searching and selecting by SUNet ID is highly recommended

- In the Project Location section, click Add Location button to Select Location Type

- A Location dialogue box will open

- For the first location, the system will default to Yes as the Primary location of the project
- Select the Location Type from the drop-down menu
- If you select Stanford Office/Lab, enter the relevant Building and Room
- You can reference the Stanford Campus Map if you are unsure of the specific building name
- If you select Stanford Office/Lab, enter the relevant Building and Room


- The page will refresh with the location that was added
- Make edits as necessary by clicking the Edit (pencil) or Delete (garbage can) icons

Admin Sponsor Details
Administrative Details
- All fields are required except the Proposal Nickname

- For Project Activities, as needed, refer to RPH 13.2 Categories of Sponsored Projects
- For On Campus / Off campus determinations, as needed, refer to RPH 15.1 Facilities and Administrative (Indirect Cost) and Fringe Benefits Rates
- For Proposal Title, enter proposal title
- Proposal Nickname is optional
- For Department Lab/Institute Submitting Proposal, enter the department where work will be conducted

Project Contacts
- In the Department Contact field, key in the first 3 letters of the contact’s SUNet ID to populate and select from the name list
- In the Department PTA Setup Contact field, key in the first 3 letters of the contact’s SUNet ID to populate and select from the name list

Sponsor Details
- Search and select the correct Sponsor/ Entity
- If you cannot find your sponsor/entity, submit a SeRA Help Ticket to have the sponsor/ entity added to the SeRA sponsor/ entity table.

- Limited Submission - Indicate (Y/N) if the funding opportunity limits the number of applications that can be submitted from an institution. Not Sure? Please refer to the Limited Submissions FAQ for assistance.
- If Yes, approved investigators must attach a copy of their Office of the Vice Provost and Dean of Research (VPDoR) approval letter.
- Contact limitedsubmissions@stanford.edu with any questions about university-wide limited submission programs or the internal application process
- Limited submission programs with a clinical or biomedical research focus are facilitated by the Research Management Group on behalf of the School of Medicine, additional information is available here. Please contact rmg_communications@stanford.edu if you have questions regarding clinical or biomedical programs
- Indicate (Y/N) if there is a sponsor deadline
- If Yes, enter the sponsor deadline date, time, and relevant time zone. The PDRF will calculate the Internal Deadline = the date by which the complete proposal must be received by 9am by the relevant institutional official to be considered on time. As needed, refer to Stanford’s Internal Proposal Deadline Policy
- Select the appropriate submission method from the dropdown menu
- If the submission method will be paper or email [by your Institutional Official] provide the requisite sponsor contact name & contact information

- Indicate (Y/N) if this proposal is in response to a solicitation (e.g., Program Announcement, RFP, BAA, FOA etc.)
- If yes, enter opportunity #, copy/paste URL, or attach guidelines (Category: Program Guidelines, Subcategory: Program Guidelines).
- You can also attach the guidelines by using the attach guidelines link

- Indicate (Y/N) if the sponsor imposes any restrictions on non-U.S. citizen participation.
- Indicate (Y/N) if the sponsor imposes restrictions on publication or dissemination of research results

Budget Questions
Budget Information
- LEAVE BLANK for the total Amount Requested for this proposal
Indirect Costs (IDC)
- Enter 0 for the Requested IDC rate
- Indicate Yes for ISC applies
- In accordance with Administrative Guide Memo 37.3, the Infrastructure Charge(ISC) of 8% is required on non-government sponsored project funds that carry on F&A rate of 0%.
- Indicate No if ISC is included in the proposal budget (to be paid by Sponsor)
- Indicate Yes if ISC will be covered by a Department or School
- Enter TBD@ time of award when asked to provide a non-restricted PTA

Budget Details
- Enter Start date for Budget Period 1, then click on Auto Fill, system will auto-populate start and end dates
- Enter Stipend amount for each year of the project
- Enter Institutional Allowance for each year of the project
- Enter Other Costs in Other for each year of the project
- Tuition request would be entered in this field

Project Questions
Compliance Information
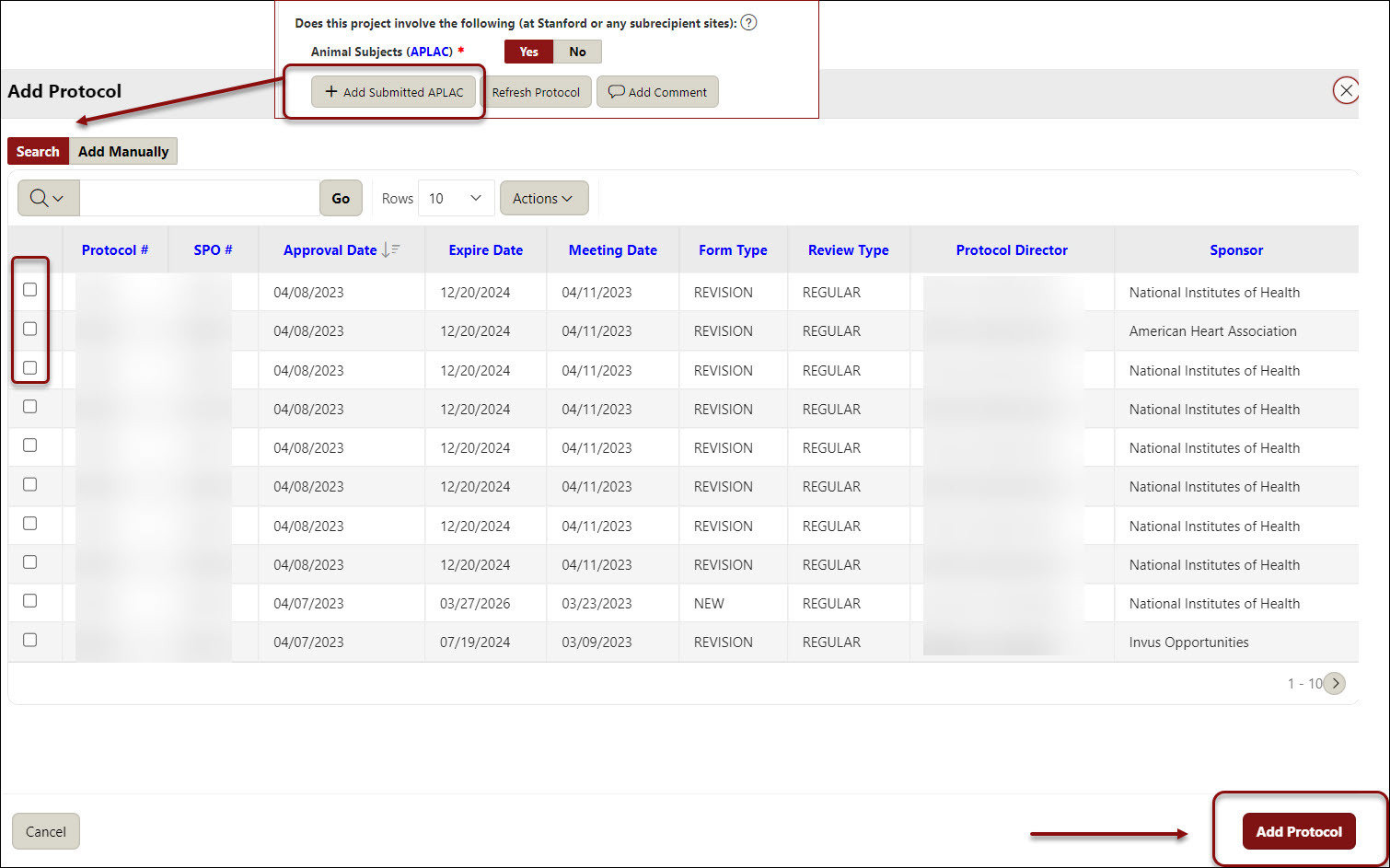
- Indicate (Y/N) if this project involves any of the following at Stanford or subrecipient sites:
Animal Subjects (APLAC)
- If yes, click the Add APLAC button, search and select the appropriate protocol(s), and click Add Protocol
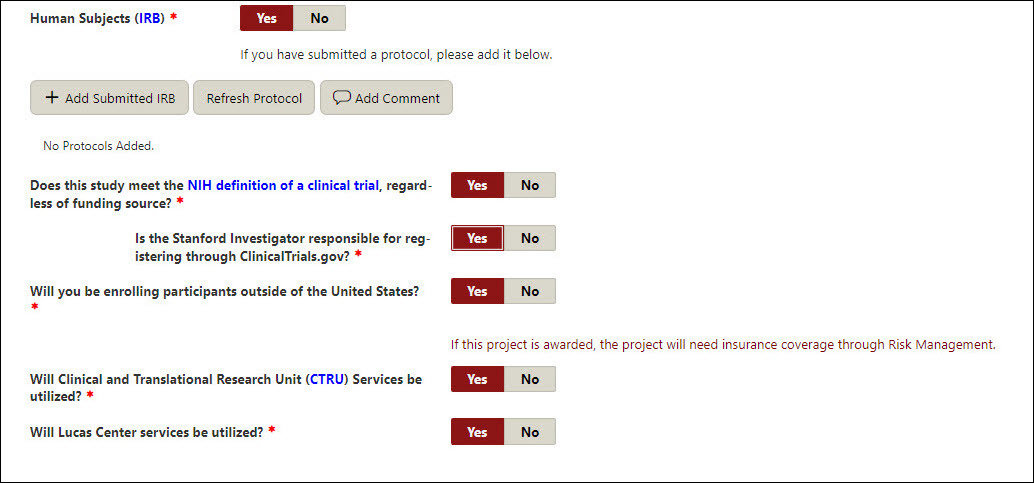
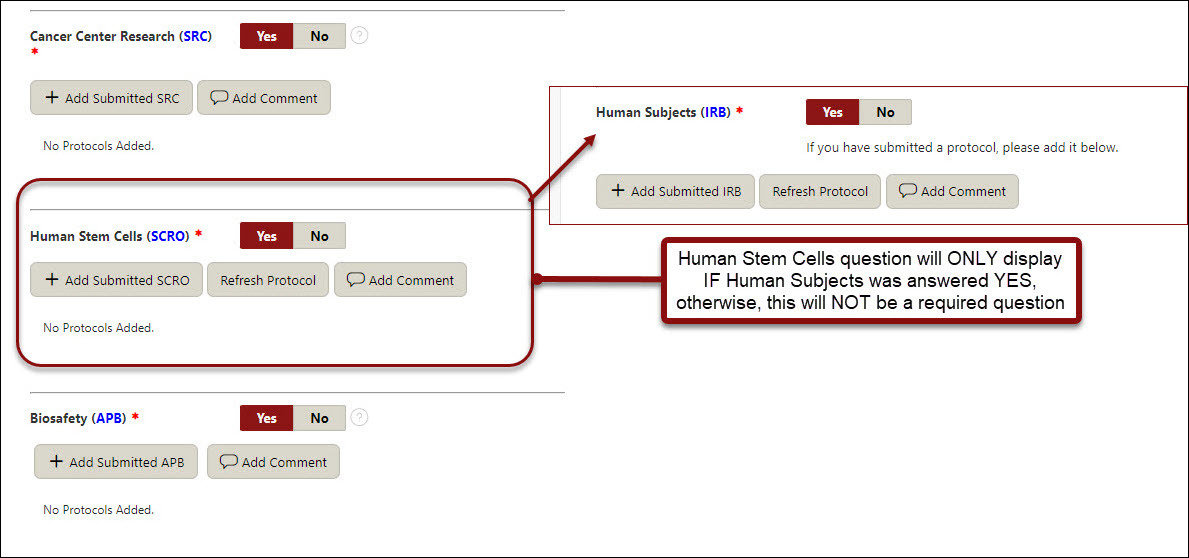
Human Subjects (IRB)
- If yes, click the Add IRB button, search and select the appropriate protocol(s), and click Add Protocol.
- Additionally, answer
- Does this study meet the NIH definition of a clinical trial, regardless of funding source?
- If yes, answer:
- Is the Stanford Investigator responsible for registering through ClinicalTrials.gov?
- If yes, answer:
- Will you be enrolling participants outside of the United States?
- If yes, and if this project is awarded, the project will need insurance coverage through Risk Management
- Budget & Billing Workbook used for patient care costs?
- If yes, a billing & budget workbook is required
- Attach workbook (Category: Detailed Budget & Justification, Subcategory: Budget & Billing Workbook)
- If yes, a billing & budget workbook is required
- Will Clinical and Translational Research Unit (CTRU) Services be utilized?
- Will Lucas Center services be utilized?
- Does this study meet the NIH definition of a clinical trial, regardless of funding source?
- Cancer Center Research (SRC) (conditional - only shows if Human Subjects are marked yes)
- If yes, click Add SRC button, and check off the appropriate protocol, then click Add Protocol
Human Stem Cells (SCRO)
- If yes, click Add SCRO button, check off the appropriate protocol, click Add Protocol
Biosafety
- The Administrative Panel on Biosafety (APB) is an established institutional biosafety committee that reviews projects involving infectious agents, recombinant DNA (rDNA), and synthetic nucleic acid molecules. The review includes safety protocols regarding the below activities:
- Safety of subjects, research personnel and the environment when using biohazardous materials and infectious agents
- Safety protocol while using infectious agents or biohazardous materials
- Human Gene Transfers
- Recombinant RNA/DNA
- If yes, click Add Submitted APB button, check off the appropriate protocol, click Add Protocol
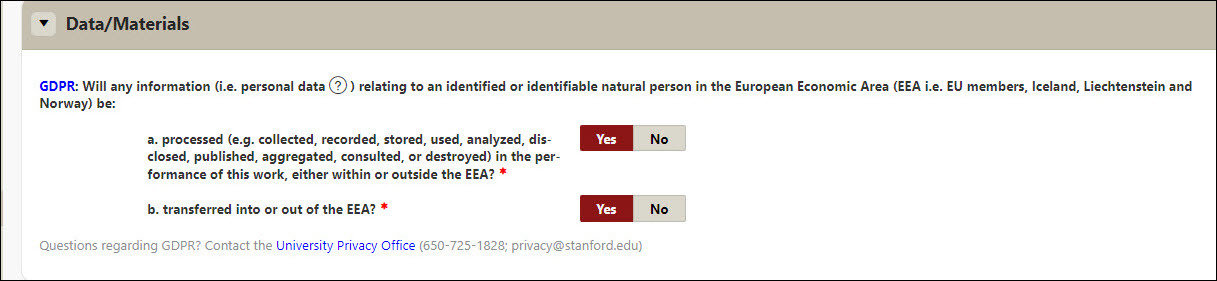
Data/Materials
- GDPR - Indicate (Y/N) to Will any information (i.e. personal data ) relating to an identified or identifiable natural person in the European Economic Area (EEA i.e. EU members, Iceland, Liechtenstein and Norway) be:
- Processed (e.g. collected, recorded, stored, used, analyzed, disclosed, published, aggregated, consulted, or destroyed) in the performance of this work, either within or outside the EEA?
- Transferred into or out of the EEA?
- Questions regarding GDPR? Contact the University Privacy Office (650-725-1828; privacy@stanford.edu )
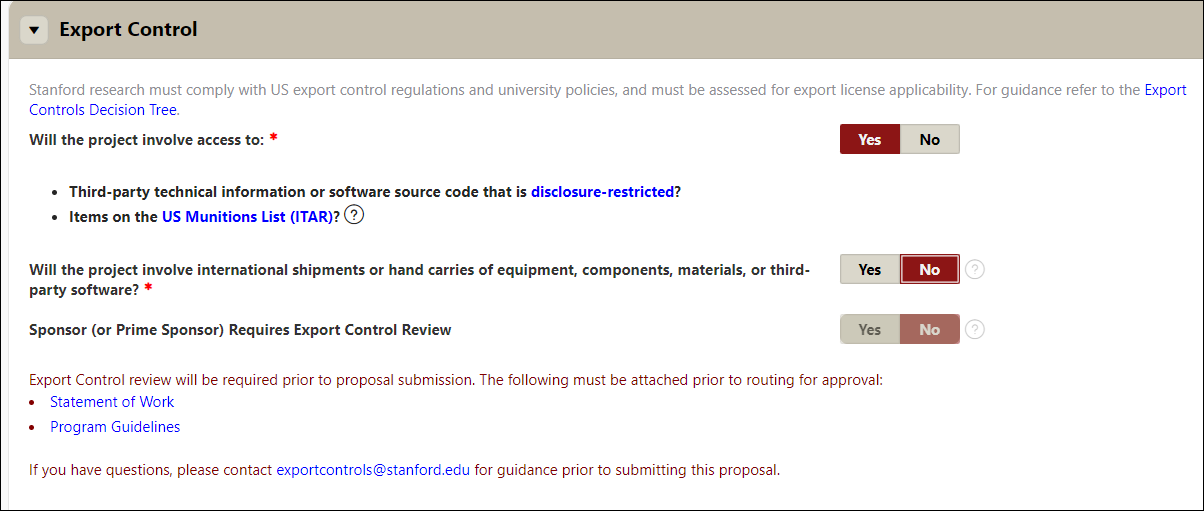
Export Control
- Stanford research must comply with US export control regulations and university policies and must be assessed for export license applicability. For guidance refer to the Export Controls Decision Tree and/or contact the VPDoR Export Control Team
- Indicate (Y/N) if the project involve access to:
- Third-party technical information or software source code that is disclosure-restricted and/or
- Items on the US Munitions List (ITAR)?
- Indicate (Y/N) If the project involves international shipments or hand carries of equipment, components, materials, or third-party software. Stanford policy requires all international shipments and Stanford-owned or loaned property hand carried abroad to be documented for export control compliance. Use the Export Controls Decision Tree for property exports and/or see DOR’s Temporary Exports Page for Stanford documentation requirements for items such as Stanford laptops taken on short-term international travel
International & Global Business
- Stanford’s Global Business Services (GBS) provides compliance and operations support for activities abroad including sponsored research activities. To more effectively serve and support projects which have global infrastructure and regulatory compliance components indicate (Y/N) if there will be any international activity as part of this project, including travel, subawards or the use of resources (e.g., funding, people, equipment, etc.) outside of the United States. For guidance refer to the Global Activity Guide.
- If yes, select the applicable countries, and answer the subsequent International Presence and Spending questions

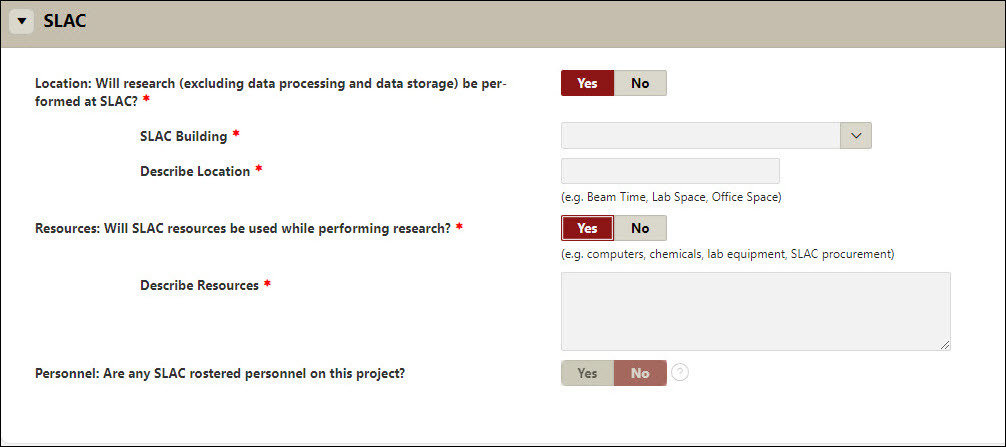
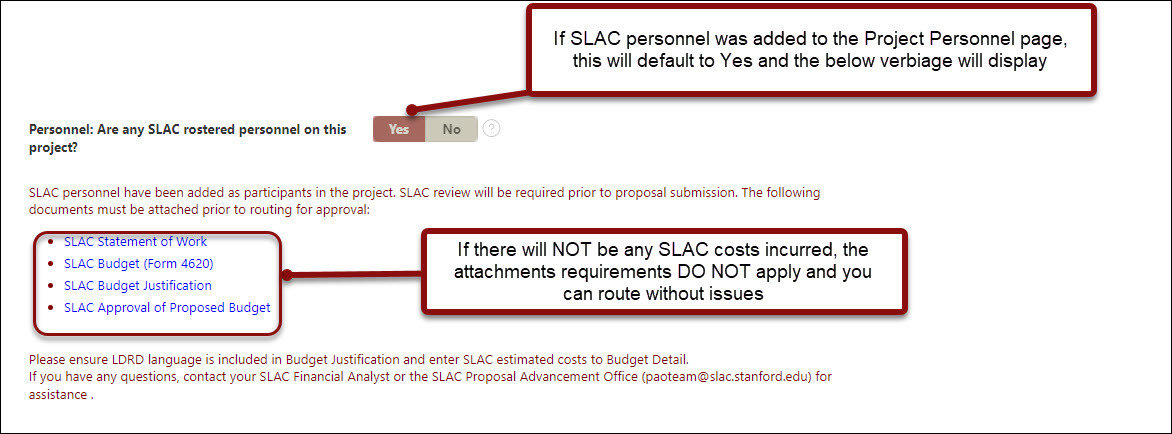
SLAC
- The Stanford Linear Accelerator Center (SLAC) National Accelerator Laboratory is a U.S. Department of Energy Office of Science laboratory operated by Stanford University. The use of SLAC resources for research activities requires review and approval by SLAC and Stanford administrative departments.
- Answer the various questions outlined specifically to SLAC
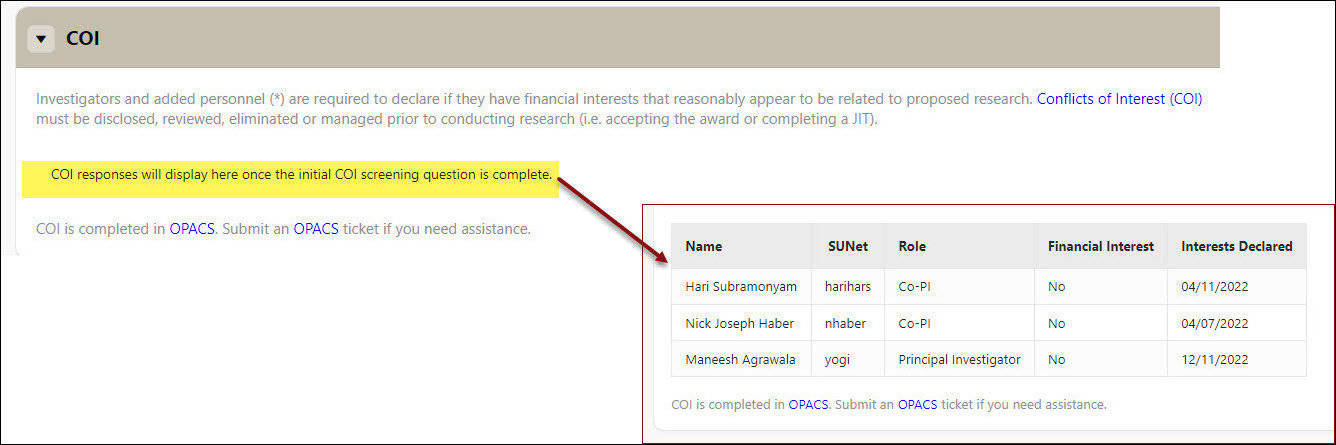
COI (Conflicts of Interest)
- Investigators and added key personnel are required to declare if they have financial interests that reasonably appear to be related to proposed research. Conflicts of Interest (COI) must be disclosed, reviewed, eliminated or managed prior to conducting research (i.e., accepting the award or completing a JIT)
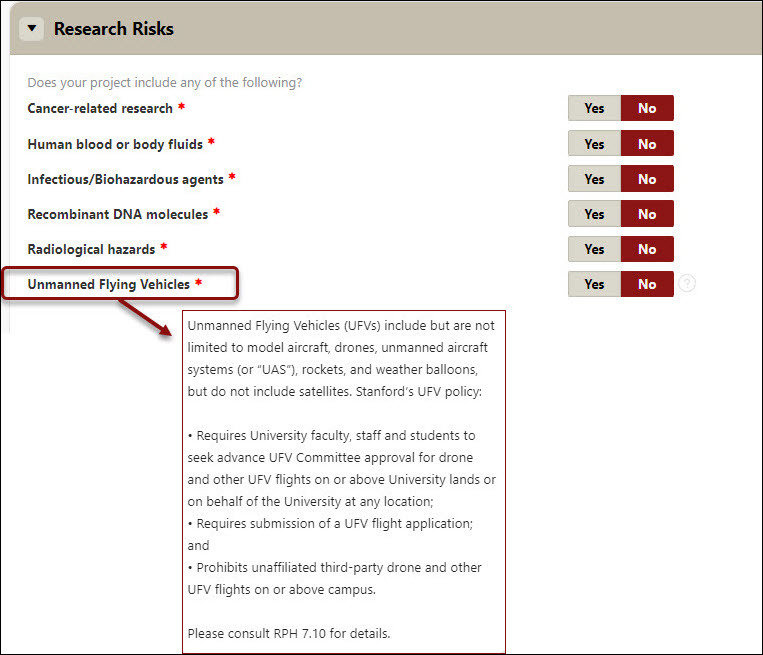
Research Risks
- Indicate (Y/N) if the project includes cancer related research, use of human blood, body fluids, infectious/biohazardous agents, recombinant DNA molecules, radiological hazards, and/or unmanned flying vehicles
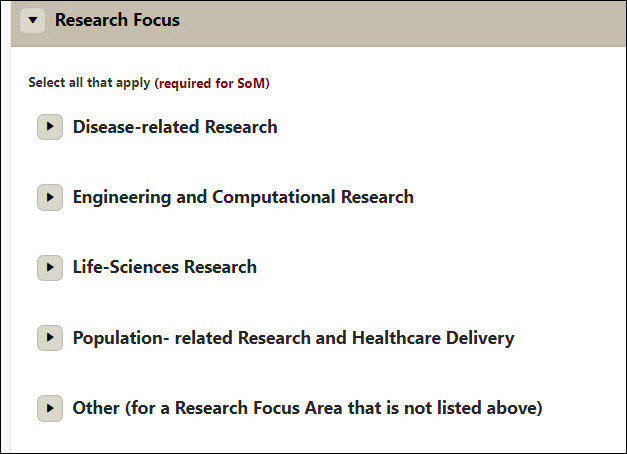
Research Focus
- Select all that apply (required for SoM)
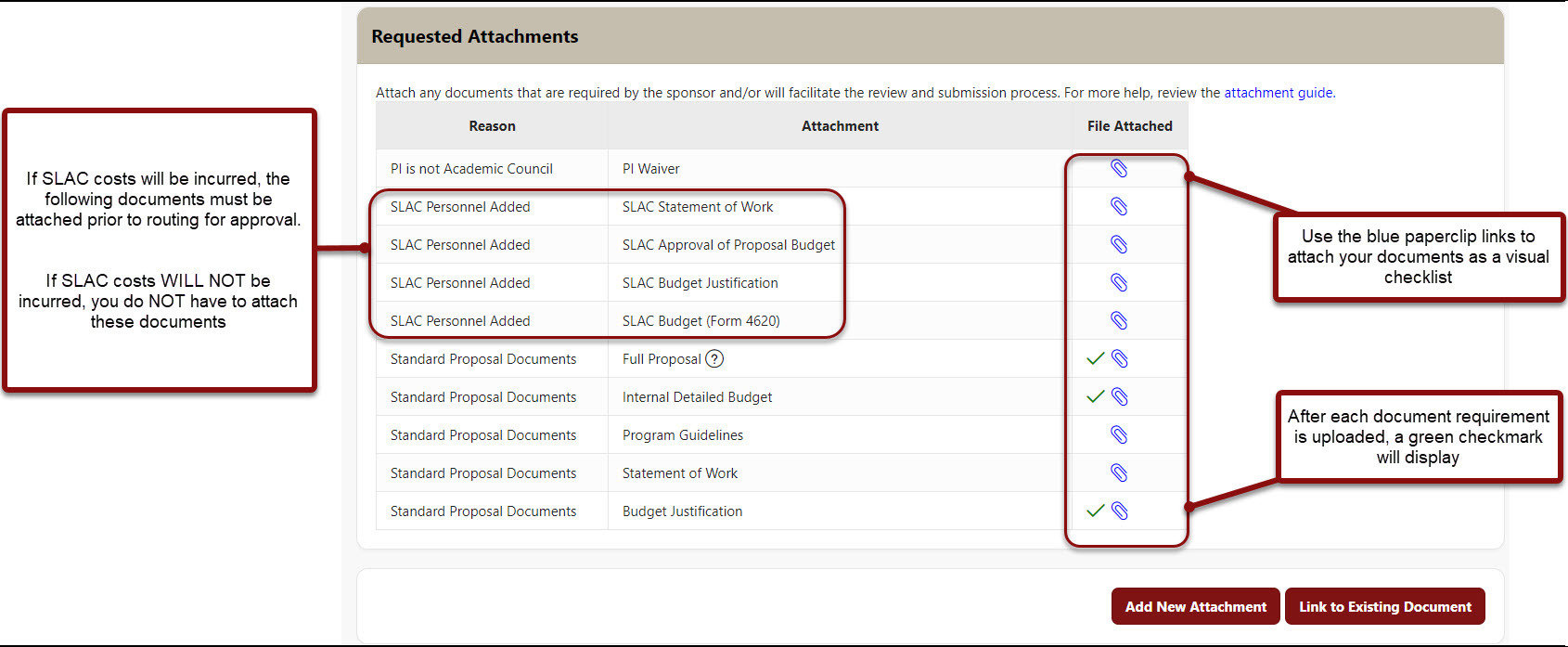
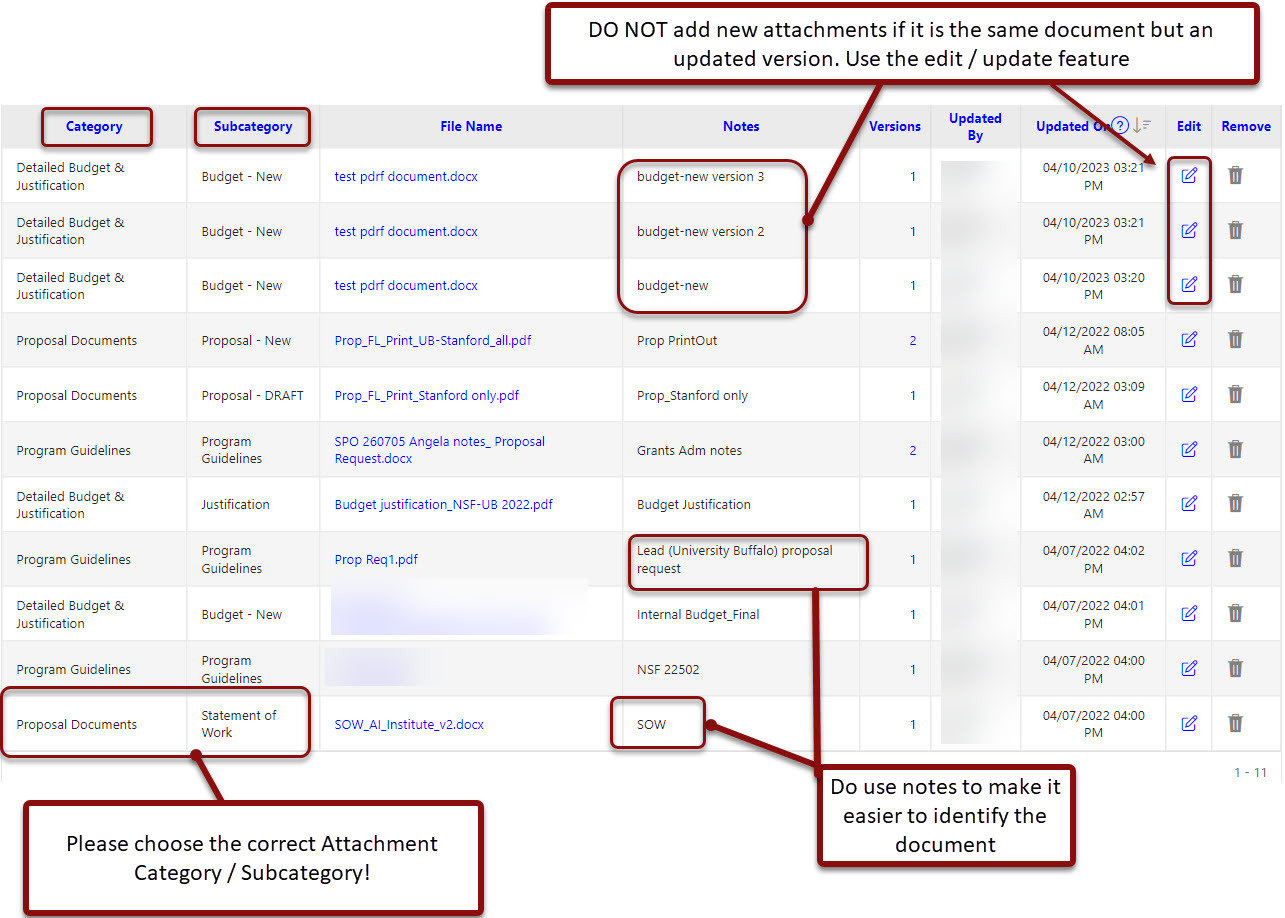
Attachments
- You MUST Attach to the PDRF
- Program guidelines
- Full proposal*
- *For proposals prepared in Cayuse or an external sponsor portal from which your institutional official will review and submit the proposal to the sponsor such as NSF Research.gov, NASA NSPIRES, etc., you do NOT need to attach a print out/PDF of the full proposal here. For external sponsor portals, as needed, ensure the appropriate access to the proposal has been provided to your institutional official. Once an institutional official submits a proposal from Cayuse or an external sponsor portal, they will print to PDF the full proposal they submitted and attached that to the PDRF record.
- Internal Detailed Budget
- Budget Justification
- Any other documents that are required by the sponsor, Stanford, and/or will facilitate the review and submission process on the Proposal & Attachments page. Use this as your electronic filing cabinet for the proposal!
- Use the blue paperclip links to attach your documents as a visual checklist. After each document is uploaded, a green checkmark will be added to each requirement
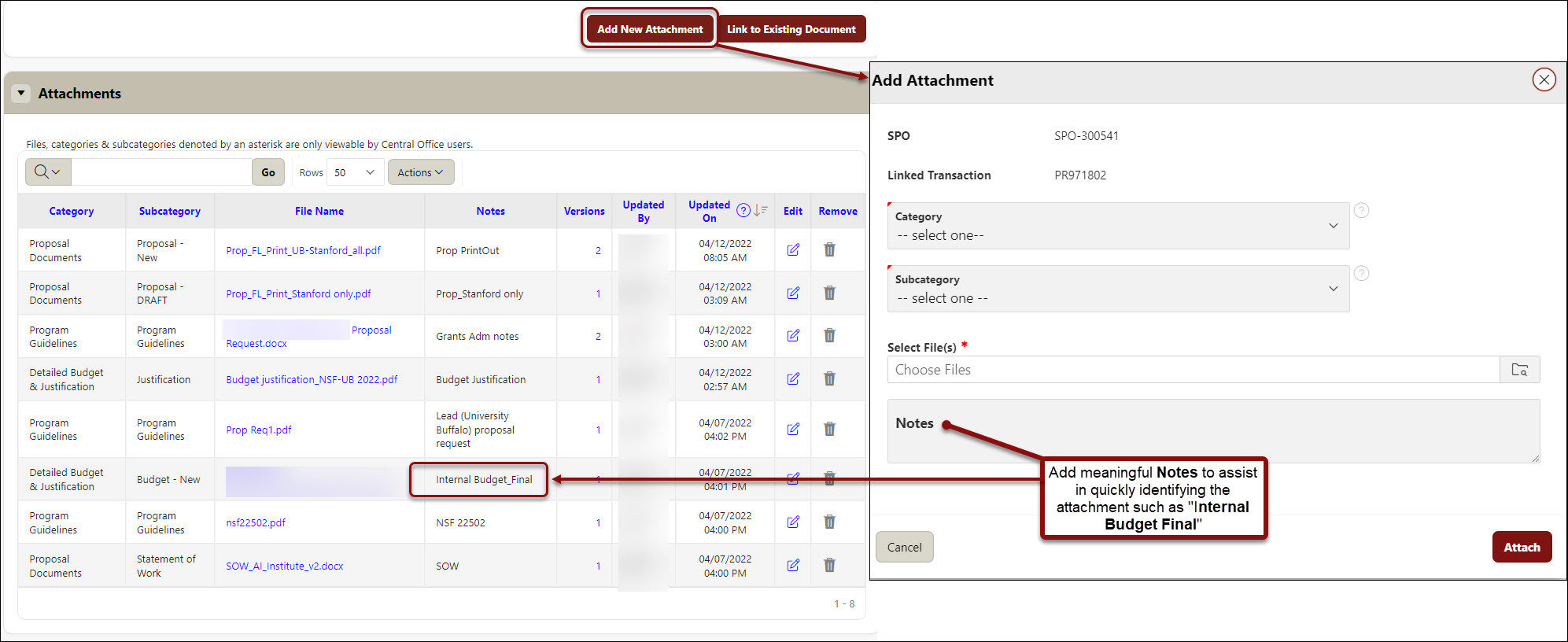
- Additional attachments than those requested can also be attached from the Attachments screen of the PDRF.
- Click on Add New Attachment to attach each document.
- Select a Category from the dropdown menu options.
- Select a Subcategory from the dropdown menu options.
- Choose your file location.
- Enter meaningful Notes to assist in quickly identifying the attachment such as “Internal Budget Final”.
- Click Attach and repeat previous steps for each additional attachment.
Attachments Best Practices
Approvers & Comments
- In the Instructions/ Remarks free text section enter any pertinent information for approvers and/or your institutional official
- Approvers - Default approvers for the PI's department are listed in the Approvers section
- Add additional approvers by searching and selecting names within the Name field
- PDRF approvals will route in the order listed
- Change routing order by clicking Up or Down arrows under Routing Order column, with the exception of the Mentor and Department Contact
- This can only be done for additional approvers Manually Added

- FYIs - Default FYIs for the PI's department will be listed if applicable. A notification will be sent when all approvals are complete. Review, add, delete, and update as needed. Add additional FYIs by searching and selecting names from the name field.
- Institutional Organization - Select School of Medicine’s Research Management Group
- Institutional Official - Select Jonathan Gagante from the dropdown menu

Emails
Have you ever saved an email and then uploaded it to a SPO, RRA, or transaction in SeRA?
This process just became easier! Now you can just send an email to SeRA (with some added “tags” in the subject line), and the SeRA system will automatically attach your email (and any attachments in the email) to the appropriate project or agreement in SeRA.
Go directly to Sending Emails to SeRA for further instructions.
Review for Completeness Routing
- Click on Review for Completeness from the right-hand navigation Actions menu
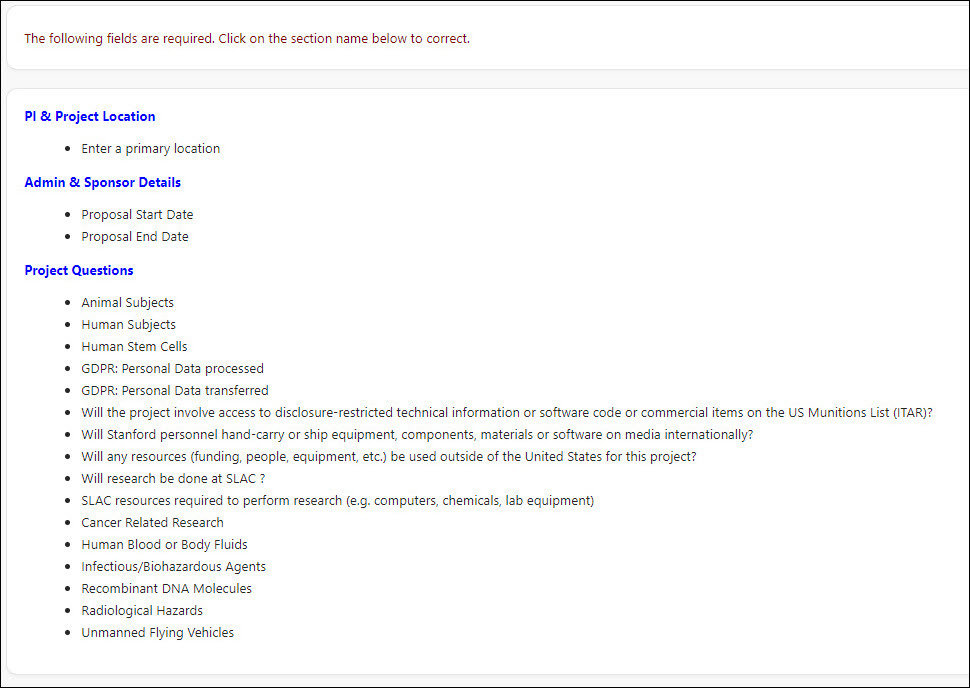
- Blue hyperlinks for the pages with incomplete required fields will be listed. Complete all required fields. Once all required fields are complete, route the PDRF for approvals
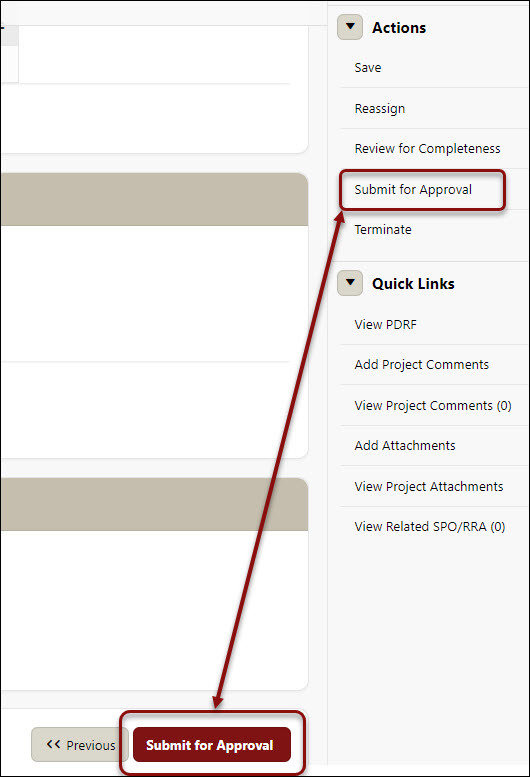
- Click the Submit for Approval either at the bottom of the page, or on the right-hand navigation menu
- You will return to your SeRA home page once the PDRF is routed. The PDRF will no longer be listed among your Action Items
- Monitor the PDRF from My Pipeline

- To view the PDRF routing status, click on the blue PDRF ID# hyperlink, and scroll to the bottom of the Transaction Home page, to Task History
