Initiate a Non Competing Continuation (NCC) / Progress Report PDRF
Scroll through the below for information on the Non Competing Continuation/Progress Report PDRF, or use the quick links to jump to a specific PDRF page's guidance.
- Initiate a Non Competing Continuation/Progress Report PDRF
- PI & Project Location
- Admin & Sponsor Details
- Project Personnel
- Budget Questions
- Budget Details
- Project Questions
- Attachments
- Approvers & Comments
- Review for Completeness & Routing
Initiate a Proposal - Non Competing Continuation/Progress Report (PDRF)
- Using your SUNet ID and passphrase log into the Stanford Electronic Research Administration (SeRA) system at https://sera.stanford.edu/
- From the red banner at the top of any page within SeRA click on Search
- Enter the SPO # for which you’d like to initiate the NCC/Progress Report PDRF
- The search will interactively display matches according to what is being entered
- Click on the appropriate blue reference number to drill into the relevant record.
- Enter the SPO # for which you’d like to initiate the NCC/Progress Report PDRF

- From the Project Summary page click on the appropriate award Segment with which the NCC/Progress Report proposal should be associated

- From the award Segment Summary page, locate the teal Start Transaction button and select Proposal – NCC/Progress Report

- A Start Transaction window will open
- Select the budget period associated with this Progress Report, and click Start

- The page will refresh to a Principal Investigator & Project Location (Proposal - Non Competing Continuation/Progress Report) page

- Proceed with completing the NCC/Progress Report PDRF by navigating through the pages using the Next or Previous buttons on the bottom right hand side of the screen, or the left hand navigation (LHN)

PI & Project Location
Principal Investigator
- The Principal Investigator (PI) field will already be pre-filled in for you
- Select the Appointment Type of the PI and enter the % of effort they will commit to this project. Include both effort that will be direct charged as well as any that is being proposed as cost share. If Academic/Summer is selected, a number, even if 0, must be entered in both the academic and summer effort boxes

- Indicate (Y/N) if ALL the above listed PI effort commitment will be direct charged to the project
- If the PI's effort will be accounted for via a combination of direct charging and cost sharing, answer No
- If No, indicate how the PI’s effort will be accounted for from the dropdown menu
- If the PI's effort will be accounted for via a combination of direct charging and cost sharing, answer No
- If you choose Other a free text comment box will open

PI Effort Note
Stanford requires a commitment of effort on the part of the PI on all sponsored projects with the following exceptions. This requirement applies even if a sponsor does not require a commitment of effort on the part of the PI and/or does not allow the direct charging of PI salary. PI effort may be expended during the academic year, summer quarter only, or both.
Stanford tracks and manages effort primarily through direct salary charges to sponsored projects, cost sharing salary charges, or a combination of direct charging and cost sharing.
The requirement of PI effort does not extend to:
- Equipment grants
- Seed grants for students/postdocs where the faculty mentor is named as PI, dissertation support, training grants, or other awards intended as "student augmentation “
- Limited-purpose awards characterized by Stanford as Other Sponsored Activities, including travel grants, conference support, etc.
See DoResearch Faculty Effort Topic page for more information
Project Location
- If the location(s) is/are different than what is already listed, click the Add Location to list the locations where work for this project will be conducted

- A Location dialogue box will open
- For the additional locations, the system will allow you to toggle Yes or No as the Primary location of the project
- Select the Location Type from the drop-down menu
- If you select Stanford Office/Lab, enter the relevant Building and Room
- You can reference the Stanford Campus Map if you are unsure of the specific building name
- If you select Stanford Office/Lab, enter the relevant Building and Room

- Indicate (Y/N) if all above space is assigned to the listed PI or otherwise approved for his/her use
- Indicate (Y/N) if you anticipate rental space, construction or renovations will be required to house this project or any equipment acquired for this project
- Click Add

- The page will refresh with the location(s) that was added
- Make edits as necessary by clicking the Edit (pencil) or Delete (garbage can) icons
- Attach department chair approval using link, if necessary

Admin & Sponsor Details
- Verify that all of the previously entered information is correct, and update as necessary
- All fields are required except the Proposal Nickname
- For Project Activities, as needed, refer to RPH 13.2 Categories of Sponsored Projects
- For On Campus / Off campus determinations, as needed, refer to RPH 15.1 Facilities and Administrative (Indirect Cost) and Fringe Benefits Rates

Project Contacts
- In the Department Contact field, key in the first 3 letters of the contact’s SUNet ID to populate and select from the name list
- In the Department PTA Setup Contact field, key in the first 3 letters of the contact’s SUNet ID to populate and select from the name list


Sponsor Details
- The Sponsor will already be listed
- Limited Submission - Indicate (Y/N) if the funding opportunity limits the number of applications that can be submitted from an institution. Not Sure? Please refer to the Limited Submissions FAQ for assistance.
- Contact limitedsubmissions@stanford.edu with any questions about university-wide limited submission programs or the internal application process
- Limited submission programs with a clinical or biomedical research focus are facilitated by the Research Management Group on behalf of the School of Medicine, additional information is available here. Please contact rmg_communications@stanford.edu if you have questions regarding clinical or biomedical programs
- Indicate (Y/N) if there is a sponsor deadline
- If Yes, enter the sponsor deadline date, time, and relevant time zone. The PDRF will calculate the Internal Deadline = the date by which the complete proposal must be received by 9am by the relevant institutional official to be considered on time. As needed, refer to Stanford’s Internal Proposal Deadline Policy

- Select the appropriate submission method from the dropdown menu
- If the submission method will be paper or email [by your Institutional Official] provide the requisite sponsor contact name & contact information

Project Personnel
Stanford Investigators
- If necessary, click Add Investigator to add each participating Investigator.

- All individuals listed in this section must approve their participation in the project and certify the completeness and accuracy of their proposal contents (and in accordance with sponsor guidelines and policies).
- Individuals listed on applications as Senior or Key Personnel with the role of Other Significant Contributor that have no effort commitment on the project should NOT be listed here.
- If desired, they can be added in the Additional Personnel section.
- Complete the Investigator Details fields as advised on screen

Effective Dates
The effective dates will reflect the time this investigator participated (or plans to participate) on this project. Please set these dates as follows:
- If the investigator is actively working on the project, leave the end date field blank.
- If the investigator is no longer working on the project, enter an end date based on when their project participation stopped.
- If the investigator re-joins the project, add a new effective date range with a new start date and click Save.

Project Location
Add the Project Locations as directed in the PI & Project Location section
Approvers
All default approvers for this investigator based on the information provided above and are listed. Review and update these approvers as needed. Routing will occur in the order specified.
- For Approvers for each Investigator, default approvers are listed based on the org code provided
- Please review the listed approvers and make updates as needed. Routing will occur in the order listed.
- Other approvers should be added as appropriate

Approval Notifications
Any default FYIs for the Investigator’s department will be listed here
- Approval Notifications will be sent when all approvals are complete
- Any Investigator’s approvals that are NOT secured prior to the overall PDRF routing will prevent the PDRF from routing to the relevant Institutional Office.

- Click Next to return to the Project Personnel main page

- Click the ROUTE button to initiate the approval routing process for each Investigator listed
- Click the pencil and garbage can icons as necessary

- A dialog box will open confirming you wish to route the Investigator approval action item. Click Route Investigator

- You will return to the Project Personnel main page where you can add additional project personnel as well as monitor the status of any routed Investigator approvals

- Navigate to the Transaction Home page to verify date/timestamps of Assigned, Completed status and you can Reassign from Transaction Home as well

Additional Personnel
- Add any non-key Stanford personnel, SLAC rostered personnel (paid by SLAC), or subrecipient personnel who are budgeted on this project by clicking on Add Personnel. For Clinical trials, the study coordinator should be included.

- Indicate if this individual is from Stanford or another institution
- Select the individual’s project Type/ Role
- Stanford (internal)
- Select the appropriate type/ role
- If the person is still to be determined, check off the box
- Key in the first 3 letters of the personnel’s SUNet ID to populate and select from the name list
- Enter the percentage of effort the person will devote to the project and click Next to navigate back to the Project Personnel main page

- Other
- To add non-Stanford personnel (external) not showing as a selection, click on the blue hyperlink to add them to the database, then click the plus sign and fill in the fields as required


- Key in the first 3 letters of the personnel’s SUNet ID to populate and select from the name list
- If the person is still to be determined, check off the box
- Enter the percentage of effort the person will devote to the project and click Next to navigate back to the Project Personnel main page
- Verify the information is displaying correctly. Use the Edit pencil or garbage can icon to update/ change the information if necessary
Budget Questions
Budget Information
- The total Amount Requested for this proposal is already pre-filled in
- Indicate (Y/N) if the sponsor has a salary cap
- If the National Institutes of Health (NIH) was selected as the sponsor on the Admin & Sponsor Details page, this question will appear.
- Indicate (Y/N) if the proposal requires the use of a modular budget
- Indicate (Y/N) if this proposal’s budget includes administrative salaries

Cost Share
Cost sharing represents the portion of allowable, allocable, and reasonable [direct and indirect] costs of a sponsored project not paid for by the sponsor and are instead borne by Stanford or third party. When proposals include cost sharing, it should be indicated on the Budget Questions page of the PDRF and supported by the appropriate documentation and approvals. For additional information about cost sharing refer to Stanford’s Cost Share Policy.

- Indicate (Y/N) if this proposal includes cost sharing. If it was indicated either the PI [on the PI and Project Location page of the PDRF] and/or any Project Personnel [on the Project Personnel page of the PDRF] will cost share any of their effort, Yes will already be selected, and a note will display that "Cost Sharing effort has been selected."
- If Yes, click Add Row to provide details for each source of cost sharing including internal, external, and subrecipient sources. Add as many rows as needed. If a row is not required, delete the row using the trash can icon.
- From the Source dropdown menu select either External, Internal, or Subrecipient
- Internal: Costs will be borne by Stanford University.
- External: Cost will be borne by a non-Stanford entity that is NOT a Stanford subrecipient on the project.
- Subrecipient: Costs will be borne by a subrecipient on the project.
- From the Mandatory/Voluntary dropdown menu select if the cost sharing is Mandatory or Voluntary.
- Mandatory: Required by the sponsor and is included in the proposal
- Voluntary: Not required by sponsor but is included in proposal
- From the Type dropdown menu select if the cost sharing is cash or in-kind.
- Cash: Expenses that can be tracked on or calculated from a Stanford or subrecipient’s general ledger. Examples include, but are not limited to…
- Academic year faculty effort for non-SoM faculty with AY appointments.
- The University contribution of graduate student RAship tuition.
- Supplies
- Travel
- In Kind: The value of non-cash contributions to the project including, with sponsor approval, any relevant third-party organization’s approved federally negotiated indirect cost rate or, a rate in accordance with the Uniform Guidance. An in-kind or matching contribution made by a party other than Stanford requires documentation from the third party supporting the use of the funds as in-kind/matching and may require a certification of fair market value.
- Cash: Expenses that can be tracked on or calculated from a Stanford or subrecipient’s general ledger. Examples include, but are not limited to…
- In the Direct Cost field enter the direct costs to be cost shared
- In the Indirect Cost field enter any foregone IDC amount that should be associated with the direct cost amount entered in column E.
- The Total Cost auto-calculates by summing columns E & F. No action is needed.
- In the Guarantee PTA field for Internal Cash cost sharing rows enter the non-sponsored* PTA(s) that will be used to fund the listed cost share expenses.
- In the Describe Contribution field type for what the cost sharing row is. Examples include, but are not limited to…
- PI ##% AY sal, FB, and foregone IDC (FY## rate)
- SU covered portion of grad student tuition – 1 RA for 3 qtrs. for 3 yrs.
- Univ of X cost share (for subrecipient cost sharing)
Cost Share FAQs
- If someone has a split appointment and will be cost sharing effort, should we enter one row for the to-be cost shared effort and enter the relevant multiple guarantee PTAs listed in the PTA field, or should we enter a row for each guarantee PTA?
- Either approach is fine.
- If someone with an Academic Year (AY) appointment will cost share both AY and summer effort, and that effort will be funded from different PTAs, should we enter one row for ALL the effort to be cost shared and enter multiple PTAs in the PTA field, or should we enter a row for either each PTA or to distinguish between AY and SM effort?
- Again, either approach is fine. However, department research administrators may find the data is more value add if the AY and SM effort are entered on separate rows.
- If multiple SU individuals, most often faculty, will be cost sharing effort should each faculty have their own row, or can they be aggregated? Does the answer change if the people are different types e.g., 1 PI + 1 Co-PI vs. 1 PI + 2 grad students?
- They can be aggregated or separated. Again, department research administrators may find the data is more value add if each person for whom effort is to be cost shared is entered on a separate row.
- For proposals including cost share, what exactly must be included with the PDRF for the cost share portion of the project?
- During institutional [OSR or RMG] review of proposals including cost sharing, institutional officials are reviewing for detailed cost share budgets, budget justifications, relevant supporting documentation, and all involved departments’ approvals for any cost share commitments. If during institutional review any of the aforementioned elements are missing, the IO should inquire and request the required information, documentation and/or approvals.
- Does approval need to be obtained from all funding managers (if there is more than 1), or can a single dept manager sign off for all?
- Approval should be secured from each funding department/org providing cost share support.
- For subrecipient cost sharing, should depts. make the distinction between cash i.e. the sub could produce a ledger vs in-kind?
- Yes, the distinction should be made between subrecipient cash and in-kind cost sharing.
If you have any cost share questions, please contact the ORA Client Advocacy & Education Team. We’re here to help!
Subawards
- Indicate (Y/N) if this proposal includes subawards.
- Unsure? Refer to ORA’s Subaward Resources
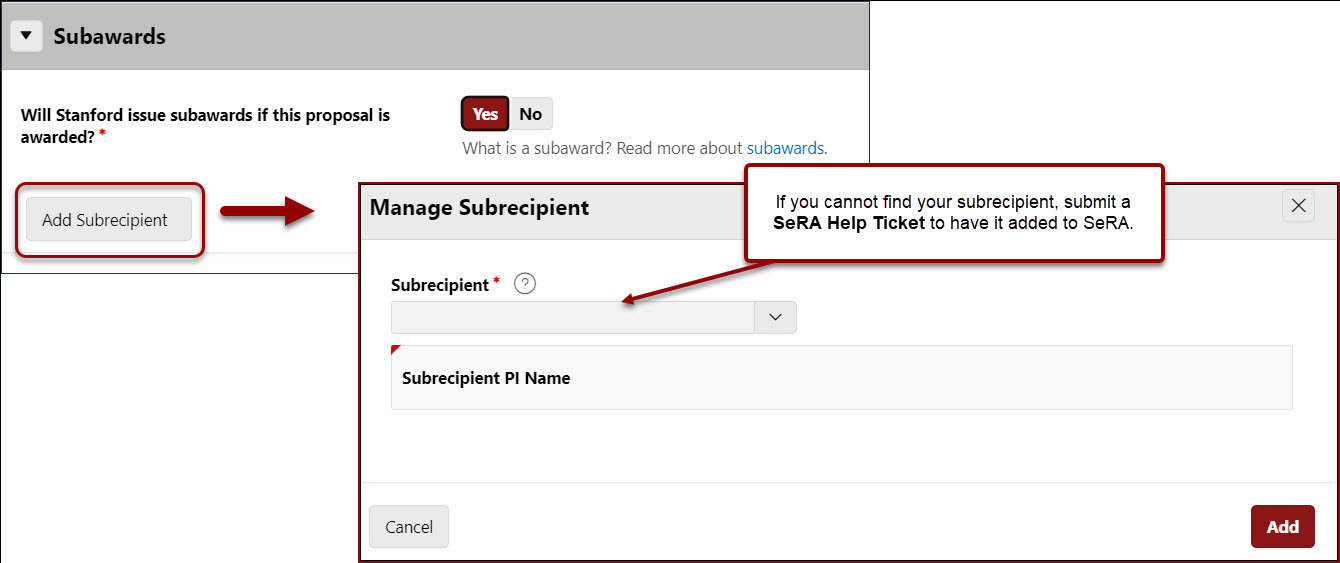
- If yes, click Add Subrecipient
- Enter the subrecipient Institution Name in the Add Subrecipient field
- If you cannot find your subrecipient, submit a SeRA Help Ticket to have it added to SeRA.
- Enter the Subrecipient PI Name
- Click Add
- Repeat previous steps for each subaward to be included in this proposal
- Click Next to advance to the Budget Details page
Budget Details
The detailed project budget will be pre-filled in based on the awarded account/PTA that has already been set up. This page can also be used to validate budgets developed in off-line spreadsheets.

Project Questions
Compliance Information
- Indicate (Y/N) if this project involves any of the following at Stanford or subrecipient sites:
Animal Subjects (APLAC)
- If yes, click the Add APLAC button, search and select the appropriate protocol(s), and click Add Protocol

Human Subjects (IRB)
- If yes, click the Add IRB button, search and select the appropriate protocol(s), and click Add Protocol.
- Additionally, answer
- Does this study meet the NIH definition of a clinical trial, regardless of funding source?
- If yes, answer:
- Is the Stanford Investigator responsible for registering through ClinicalTrials.gov?
- If yes, answer:
- Will you be enrolling participants outside of the United States?
- If yes, and if this project is awarded, the project will need insurance coverage through Risk Management
- Budget & Billing Workbook used for patient care costs?
- If yes, a billing & budget workbook is required
- Attach workbook (Category: Detailed Budget & Justification, Subcategory: Budget & Billing Workbook)
- If yes, a billing & budget workbook is required
- Will Clinical and Translational Research Unit (CTRU) Services be utilized?
- Will Lucas Center services be utilized?
- Does this study meet the NIH definition of a clinical trial, regardless of funding source?

- Cancer Center Research (SRC) (conditional - only shows if Human Subjects are marked yes)
- If yes, click Add SRC button, and check off the appropriate protocol, then click Add Protocol

Human Stem Cells (SCRO)
- The Human Stem Cells question will ONLY display IF the Human Subjects question was answered as YES
- If yes, click Add SCRO button, check off the appropriate protocol, click Add Protocol

Biosafety
- The Administrative Panel on Biosafety (APB) is an established institutional biosafety committee that reviews projects involving infectious agents, recombinant DNA (rDNA), and synthetic nucleic acid molecules. The review includes safety protocols regarding the below activities:
- Safety of subjects, research personnel and the environment when using biohazardous materials and infectious agents
- Safety protocol while using infectious agents or biohazardous materials
- Human Gene Transfers
- Recombinant RNA/DNA
- If yes, click Add Submitted APB button, check off the appropriate protocol, click Add Protocol

Data/Materials
- Only for NIH
- Indicate (Y/N) to Is your proposed research project subject to the 2023 NIH Data Management and Sharing Policy?
- Need more information? Visit the RMG data management & sharing webpage.
- Indicate (Y/N) to Is your proposed research project subject to the 2023 NIH Data Management and Sharing Policy?
- Indicate (Y/N) to Genomic Data Sharing: Are you creating, generating, or using large-scale genomic data?
- GDPR - Indicate (Y/N) to Will any information (i.e. personal data ) relating to an identified or identifiable natural person in the European Economic Area (EEA i.e. EU members, Iceland, Liechtenstein and Norway) be:
- Processed (e.g. collected, recorded, stored, used, analyzed, disclosed, published, aggregated, consulted, or destroyed) in the performance of this work, either within or outside the EEA?
- Transferred into or out of the EEA?
- Questions regarding GDPR? Contact the University Privacy Office (650-725-1828; privacy@stanford.edu )

Export Control
- Stanford research must comply with US export control regulations and university policies and must be assessed for export license applicability. For guidance refer to the Export Controls Decision Tree and/or contact the VPDoR Export Control Team
- Indicate (Y/N) if the project involve access to:
- Third-party technical information or software source code that is disclosure-restricted and/or
- Items on the US Munitions List (ITAR)?
- Indicate (Y/N) If the project involves international shipments or hand carries of equipment, components, materials, or third-party software. Stanford policy requires all international shipments and Stanford-owned or loaned property hand carried abroad to be documented for export control compliance. Use the Export Controls Decision Tree for property exports and/or see DOR’s Temporary Exports Page for Stanford documentation requirements for items such as Stanford laptops taken on short-term international travel

International & Global Business
- Stanford’s Global Business Services (GBS) provides compliance and operations support for activities abroad including sponsored research activities. To more effectively serve and support projects which have global infrastructure and regulatory compliance components indicate (Y/N) if there will be any international activity as part of this project, including travel, subawards or the use of resources (e.g., funding, people, equipment, etc.) outside of the United States. For guidance refer to the Global Activity Guide.
- If yes, select the applicable countries, and answer the subsequent International Presence and Spending questions


SLAC
- The Stanford Linear Accelerator Center (SLAC) National Accelerator Laboratory is a U.S. Department of Energy Office of Science laboratory operated by Stanford University. The use of SLAC resources for research activities requires review and approval by SLAC and Stanford administrative departments.
- Answer the various questions outlined specifically to SLAC


Training
- Stanford requires individuals new to the position of Principal Investigator (PI) to understand the regulatory environment in which sponsored research is conducted. The PI training course outlines the regulatory environment for sponsored research. If the PDRF shows your PI training is not current, please click on Complete your PI Training link. For any issues, please submit a STARS ticket.

COI (Conflicts of Interest)
- Investigators and added key personnel are required to declare if they have financial interests that reasonably appear to be related to proposed research. Conflicts of Interest (COI) must be disclosed, reviewed, eliminated or managed prior to conducting research (i.e., accepting the award or completing a JIT)

Research Risks
- These answers have been pre-populated based on the previous award
- Update (Y/N) answers as appropriate if the project includes cancer related research, use of human blood, body fluids, infectious/biohazardous agents, recombinant DNA molecules, radiological hazards, and/or unmanned flying vehicles

Research Focus
- Select all that apply (required for SoM)

Waivers
- The status of any waivers that have been initiated (PI eligibility, PI effort, IDC, Budget Development, IRB Fee) related to the proposal will be displayed in this section
- If necessary to add, navigate to the Transaction Log page and click on the Waivers region
- Click on the Add Waiver Type button
- Select the appropriate waiver type and enter the information as necessary


Attachments
- You MUST Attach to the PDRF
- Program guidelines
- Full proposal*
- *For proposals prepared in Cayuse or an external sponsor portal from which your institutional official will review and submit the proposal to the sponsor such as NSF Research.gov, NASA NSPIRES, etc., you do NOT need to attach a print out/PDF of the full proposal here. For external sponsor portals, as needed, ensure the appropriate access to the proposal has been provided to your institutional official. Once an institutional official submits a proposal from Cayuse or an external sponsor portal, they will print to PDF the full proposal they submitted and attached that to the PDRF record.
- Internal Detailed Budget
- Budget Justification
- Any other documents that are required by the sponsor, Stanford, and/or will facilitate the review and submission process on the Proposal & Attachments page. Use this as your electronic filing cabinet for the proposal!
- Use the blue paperclip links to attach your documents as a visual checklist. After each document is uploaded, a green checkmark will be added to each requirement

- Additional attachments than those requested can also be attached from the Attachments screen of the PDRF.
- Click on Add New Attachment to attach each document.
- Select a Category from the dropdown menu options.
- Select a Subcategory from the dropdown menu options.
- Choose your file location.
- Enter meaningful Notes to assist in quickly identifying the attachment such as “Internal Budget Final”.
- Click Attach and repeat previous steps for each additional attachment.
Attachments Best Practices

Approvers & Comments
- In the Instructions/ Remarks free text section enter any pertinent information for approvers and/or your institutional official
- Approvers - Default approvers for the PI's department are listed in the Approvers section.
- Review, add, delete, and update as needed.
- Add approvers by searching and selecting names within the Name field.
- PDRF approvals will route in the order listed.
- Change routing order by clicking the Up or Down arrows in the Routing Order section

Recommendation: add the Department Contact on the Admin & Sponsor Details page as a department approver.

- FYIs - Default FYIs for the PI's department will be listed if applicable.
- A notification will be sent when all approvals are complete.
- Review, add, delete, and update as needed.
- Add additional FYIs by searching and selecting names from the name field.

- Institutional Organization - Select either the Office of Sponsored Research or the School of Medicine’s Research Management Group

Review for Completeness Routing
- Click on Review for Completeness from the right-hand navigation Actions menu.

- Blue hyperlinks for the pages with incomplete required fields will be listed. Complete all required fields. Once all required fields are complete, route the PDRF for approvals.

- Click the Submit for Approval either at the bottom of the page, or on the right-hand navigation menu.

- You will return to your SeRA home page once the PDRF is routed. The PDRF will no longer be listed among your Action Items.
- Monitor the PDRF from My Pipeline.

- To view the PDRF routing status, click on the blue PDRF ID# hyperlink, and scroll to the bottom of the Transaction Home page, to Task History

Need further SeRA assistance? Have questions, feedback, or are experiencing other issues? Please submit a HelpSU ticket to the SeRA Support Team and a SeRA Support Analyst will contact you shortly.